28-day strengths. Addition of SO3 (as sulfate) during clinkering promotes the formation of β- belite at low levels, but α’-belite at higher additions. In these mixes C2S forms from the decompo- sition of intermediate sulfo-spurrite (2C2S •CÆ).
Stabilization of α’-C2S
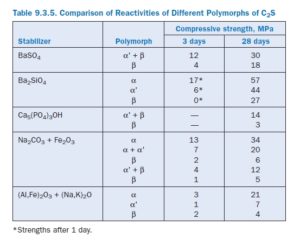
Considerable attention has been paid to the formation of cements in which α’-C2S is stabilized by the presence of foreign ions. Although α’-C2S is also a reactive phase, it is much more difficult to stabilize at room temperature. Most reactive belite cements rely on the formation of a stable α’ polymorph stabilized by an excess amount of an impurity element. A comparison of reactivities of the different polymorphs is given in Table 9.3.5. Chemical stabilization of C2S polymorphs (Ghosh and others, 1979; Moranville-Regourd and Biokova, 1992), and their relative reactivity (Stark and others, 1979; Young, 1998), have been reviewed.
However, the stabilization of high temperature polymorphs by impurity elements have physical as well as chemical mechanisms. On cooling, complex microstructures develop as a result of poly- morphic transformation (Moranville-Regourd and Biokova, 1992; Chan and others, 1992). Lamellar structures form during the α→ α’ transformation and twinning during α’ → β, while the β→γ transformation is martensitic and hence strongly dependent on crystal size and matrix restraint (Matkovic and others,1981). Stabilization of α’ by rapid cooling must be due in large part to inhibiting the nucleation of β or γ structures. Ghose and others (1983), and Chan and others (1988) observed that during cooling, exsolution of impurity atoms form second phases at grain boundaries or between lamellae (see Figure 9.3.1). These second phases can be glassy or crystalline and must play an important role in determining reactivity, since the behavior of a particular poly- morph depends on the nature of the stabilizer (Young, 1998). For a given stabilizer, α’ is always more reactive than β, but some preparations of β-C2S may be more reactive than certain α’-C2S preparations, depending on the nature of the stabilizer. Belite-rich clinkers have been prepared in the laboratory by Bobesic and others (1981), Matkovic and others (1986), and Gies and Knöfel (1986), but although some pilot scale studies have been carried out, there appear to be no belite- rich clinkers produced that do not contain calcium sulfoaluminate to provide high early strength.