Contents
Cement Kiln Liquid phase

[wpecpp name=”package + Updates forever” price=”250″ align=”center”]
UNDERSTANDING CLINKER LIQUID PHASE
INTRODUCTION
Clinker liquid phase or clinker melt is the fraction of the kiln feed that melts between the upper transition and the burning zone. The liquid has a critical role in clinker nodulization and clinker mineral development and properties. In the absence of liquid, the conversion of C2S and free lime to C3S would be almost impossible in the kiln.
Plant chemists and kiln operators are usually more concerned with the amount of liquid rather than with the rheological1 properties of the liquid. The latter is much more important during clinkering reactions than the former.
AMOUNT OF LIQUID PHASE
If the raw mix consisted of only four oxides, i.e. CaO, SiO2, Al2O3 and Fe2O3, it would start melting at 1338 oC, the so-called eutectic2 temperature for the system C-S-A-F. At the eutectic temperature, the liquid composition is 55% CaO, 6 % SiO2, 23 % Al2O3 and 16 % Fe2O3. Such composition is saturated in lime and unsaturated in silica. Therefore, it is aggressive to refractory products containing silica or silicates in their composition.
Industrial raw mix contains impurities such as MgO, Na2O, K2O and SO3. At certain concentrations, these impurities reduce the eutectic temperature of the system to 1280 oC, thus promoting earlier clinker formation. These oxides act as fluxes3 in the kiln, forming liquid as far up as in the calcining zone. Formulas used to compute the amount of liquid at any given temperature usually take into account these minor oxides. Example: % L.P. at 1450 oC = 3 x A + 2.25 x F + MgO + K2O + Na2O + SO3 (MgO2)
For most commercial clinkers, the amount of liquid phase in the burning zone varies between 23 and 29%. Higher values can be damaging to most refractory bricks in the absence of a stable coating. As the brick is infiltrated and saturated with liquid, its elastic modulus4 increases and so does its tendency to spall off
. Liquid phase calculations can be used to predict where in the kiln the stable coating will form. The amount of liquid is calculated at 1338 C and at 1450 C and the results are compared, as shown on the following chart.
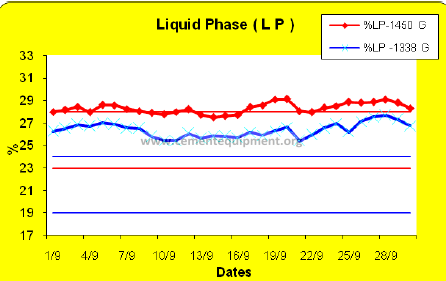
The closer the two lines are to each other, the longer will be the stable coating zone. Too much liquid at 1338 ºC is undesirable because of the proximity of the liquid to the alumina brick section. Alumina brick is quickly destroyed by clinker melt.
The tendency to coating formation or the coatability of the clinker increases with the amount of liquid. However, more coating does not necessarily mean better coating. Coating refractoriness, texture and stability are by far more important than the amount of coating deposited on the lining. A good example is the thin but stable coating encountered in white cement kilns, where the silica ratio of the raw mix is above 10 and the C4AF is 0.
IMPORTANCE OF THE LIQUID PHASE
The most important clinker mineral C3S (alite) requires the presence of liquid for its formation. In the absence of liquid, alite formation is extremely
slow and it would render commercial clinkering impossible. This fact also explains why alite is formed essentially in the burning zone, where the amount of liquid is at a maximum. To understand why alite formation requires liquid phase, one must first understand the alite formation mechanism:
1. C2S and free CaO dissolve in the clinker melt.
2. Calcium ions migrate towards C2S through chemical diffusion.
3. C3S is formed and crystallized out of the liquid.
Without liquid phase the diffusion of Ca ions towards C2S would be extremely slow, and that of C2S almost impossible, at commercial clinkering temperatures. It is important to mention that Na2O and K2O decrease the mobility of Ca ions, whereas MgO and sulphates considerably increase it. This is why the addition of gypsum to the raw mix promotes alite formation. Similarly, the addition of metallurgical slags to the raw mix promotes clinker formation. Fluxes, such as calcium chloride, feldspars and slags should not be confused with mineralizers, although both promote clinker formation. Mineralizers are usually transition metals such as copper, lead and zinc that reduce the amount of energy required for clinker silicate formation.
PROPERTIES OF THE LIQUID PHASE
VISCOSITY
Temperature has the most pronounced effect on liquid phase viscosity. Increasing the burning temperature by 93 oC reduces liquid viscosity by 70% for a regular Type I clinker. This simple fact explains why hotter-than-

normal temperatures are so beneficial to clinkering and yet so harmful to the refractory lining, as shown on the photo below. Low viscosity liquid infiltrates the refractory lining faster, leading to its premature failure.
MgO, alkali sulphates, fluorides and chlorides also reduce liquid phase viscosity. For instance, a regular clinker at 1450 ºC has a viscosity of 0.16 N.s/m2. Adding 2% SO3 to the clinker reduces that viscosity to 0.05 N.s/m2. Over-sulfated clinkers are usually dusty as a consequence. Extreme caution should be exerted when insufflating calcium chloride into the burning zone as a way to reduce alkali in the clinker. The injection of sodium carbonate into the burning zone is also detrimental to the refractory lining. Free alkali and phosphorus increase liquid phase viscosity, but this effect is offset by MgO and SO3. Only clinkers with sulphate/alkali ratio lower than 0.83, and low in MgO, would experience the negative effects of high liquid viscosity
. The liquid phase viscosity increases linearly with the alumina/iron ratio. For a given burning temperature, high C3A clinkers tend to nodulize better than low C3A clinkers. Moreover, the liquid phase is considerably less damaging to the refractory lining when the liquid is viscous.
Another important property of the liquid phase is its surface tension, or its ability to “wet” the lining. The surface tension has a direct impact on clinker fineness, coating adherence to the lining and clinker quality.
High surface tension values favor nodule formation and liquid penetration through the pores of the nodules. The resulting clinker contains less dust (fraction below 32 mesh) and lower free lime content. A liquid phase with high surface tension has less tendency to adhere to the brick surface, therefore reducing clinker coatability or adherence to the lining.
Alkali, MgO and SO3 reduce liquid surface tension. So does temperature. Sulphur and Potassium have the strongest effects, followed by Sodium and Magnesium. Therefore, MgO, SO3 and K2O, to a certain concentration, are good coating promoters.
Unfortunately, the liquid properties that induce C3S formation are detrimental to the refractory lining and to clinker nodulization.
CONCLUSIONS
Although the amount of liquid phase in the burning and transition zones of the kiln is important to clinker formation and brick performance, the rheological properties of the melt are even more important. The rheological properties of the clinker melt control parameters such as clinker mineral formation, clinker coatability, clinker fineness, cement strength and refractory depth of infiltration.
It is then very important to keep fuel, raw material properties and flame temperature as steady as possible. Whenever introducing drastic changes in raw material or fuel properties, the refractory lining must be changed accordingly to meet the differences in clinker coatability and burnability. This proves particularly true when adding slags, kiln dust or solid wastes to the kiln.
