Contents
click here to Download the Most Important 13 Books in Cement Industry
click here to Download the Most Important 13 Books in Cement Industry
EVERYTHING YOU NEED TO KNOW ABOUT Coating and Ring Formations In A Rotary Kiln
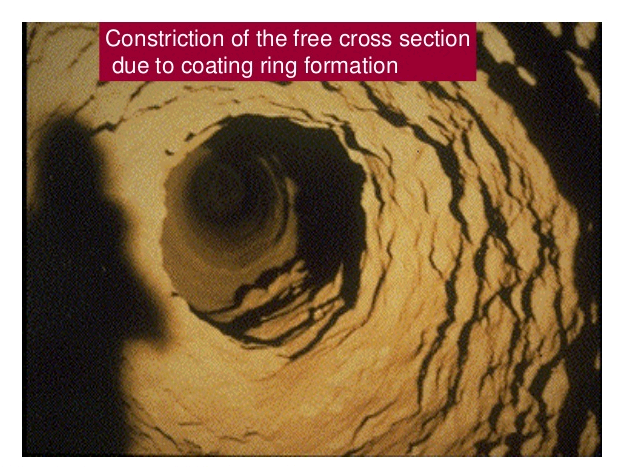

COATING FORMATION IN THE BURNING ZONE
A good protective coating on the refractory in the burning zone serves to prolong the life of the refractory. Frequent replacement of refractory costs a large amount of money, not only because of the high cost of the refractory, but also because of the loss in production while the kiln is down for lining replacement. Needless to say, frequent renewal of refractory is. an undesirable condition in any kiln.
Although refractories in the burning zone have to be replaced from time to time, a kiln operator nevertheless has the capacity to increase (and unfortunately also to decrease) the life of the lining by his ability to control the coating in the burning zone.
Nature of the Coating.
Coating is a mass of clinker or dust particles that adheres to the wall of the kiln, having changed from a liquid or semiliquid to a solidified state. The solidified particles adhere to the surface of the coating (CS in Fig. 11.1), (or the refractory surface (BS) when no coating exists), as long as the temperature of the surface of the coating is below the solidifying temperature of the particles. Coating continues to form until its surface reaches this solidifying temperature. When the kiln operates under such condition at equilibrium, the coating will maintain itself. This means that theoretically no new coating is formed. when this temperature is exceeded, however, the particles on the surface of the coating change again from a solid to a liquid state, and the coating will start to come off.
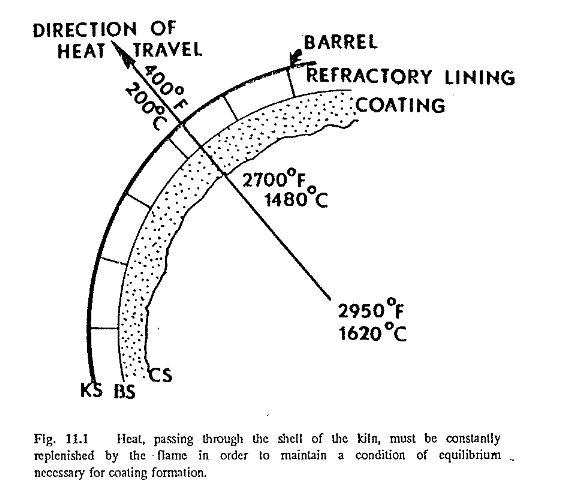
There is a temperature drop between the coating surface (CS) and the kiln shell (KS), the heat flowing in the direction indicated by the arrow in Fig. 11.1. (Heat always travels from a place or body of high temperature to a place of body of lower temperature.) This heat transfer is governed to a great extent by the conductivity of the refractory and the coating. The better the conductivity of the refractory, the better the chance of coating formation, explained by the fact that the more heat that travels in the direction of the arrow, the lower the temperature will be at the surface of the coating. Because the coating consists of particles that have changed from a liquid to a solid state, the amount that any kiln feed liquefies at clinkering temperature plays a very important role in coating formation. A kiln feed with a high liquid content at clinkering temperatures is more effective in coating formation than a feed low in liquid. Kiln feeds with a high liquid phase (easy-burning mixes) have a high content of fluxes: the iron, alumina, magnesia, and alkalies. On the other hand, hard-burning mixes (low in iron, alumina, magnesia, and alkalies, and high in silica and lime) donot have a favo able influence on coating formation. Alkalies entrained in the gas stream promote the formation of coating (unfortunately rings also) because of their high fluxing characteristics.
Because the surface temperature is probably the most important factor in the formation of a coating, it is obvious that the flame itself has a significant effect on coating formation because the shape of the flame directly governs the surface temperature at any given point in the burning zone. A flame that is too short, snappy, and wide can erode the coating because of the great heat released over a short area with this kind of flame. A long flame is more favorable to coating formation in·the burning zone. In Chapter 6 it was pointed out that short flames are desirable for better control of the burning operation, but the flame should be shortened only to the extent that it will not harm the coating.
Once all the factors have been taken care of and the foundation for a good protective coating established, it is then up to the kiln operator to control the coating during operation of the kiln. It is his responsibility to form and maintain a good solid coating in the burning zone.
Operating Conditions.
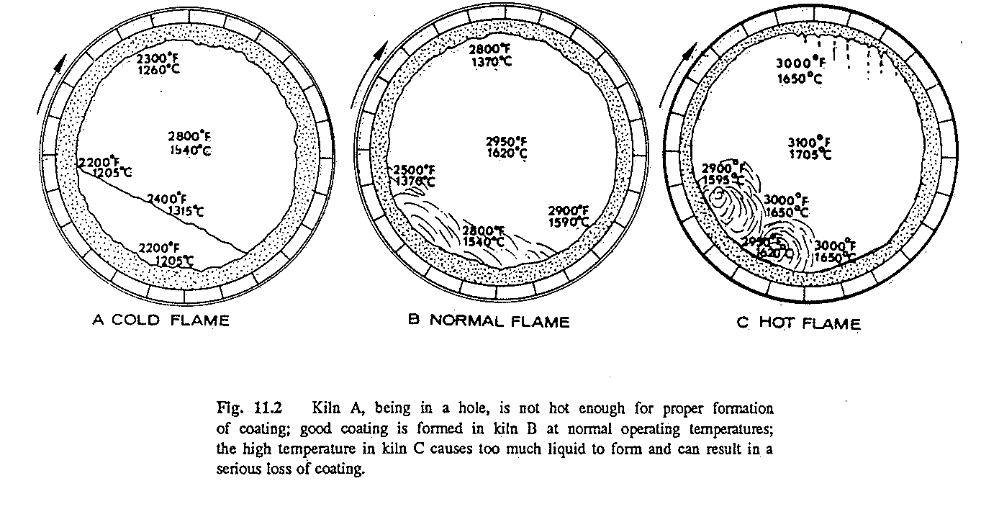
Operating conditions are just as vital for coating formation as all the other factors mentioned above. Assume that a kiln will be operated from one extreme of temperature to the other, that is, a cold, a normal, and a badly overheated kiln; that the same kiln-feed composition is burned in all three examples; that the solidifying temperature is 2400 F (1315 C); and that 24% liquid is formed at the point of investigation, under ideal operating condition.
First, consider the cold kiln (Fig. 11.2A). In this case almost no coating is formed. The coating surface temperature as well as the feed temperature is too low to produce the necessary amount of liquid matter that would promote coating formation. The condition in this example is commonly referred to by kiln operators as the kiln being in a “hole.” This example also supports the widely known fact that no new coating can be formed while the kiln is cold.
In the normal kiln (Fig. I I.2B), enough liquid (24%) is present to form a coating. Temperature of the coating when it emerges from the feed bed, as well as when in contact with the feed, is below the solidifying temperature of the feed particles. The particles will adhere to the wall and solidify, and will continue to do so as long as the surface temperature of the coating remains below the solidifying temperature of 2400 F (1315 C). Whenever the wall reaches this temperature no new coating will form. The coating is in equilibrium.
In the hot kiln (Fig. I I.2C), because of the extremely high temperatures of the feed and the coating, too much liquid is formed. As all temperatures are above the solidifying temperature, the coating transforms from a solid back to a liquid again. In such a condition, coating will come off, and the feed because of its high liquid content will “ball up.” Needless to say, this condition is extremely harmful to the kiln and to the refractory.
Most basic refractories, and especially the dolomite liners, are not able
to withstand prolonged exposure to the high flame temperatures without this protective coating. As was mentioned in the previous chapter, the burning zone is divided into three subzones namely the upper-transition 1 the sintering, and the lower-transition zones. Because of the lower liquid content in the feed and because of the frequent temperature changes, the upper- and lower-transition zones are areas where formation and maintenance of coating is the most unstable. Shifting burning zone locations produce a similar shift in the location where coating is formed; thus, unstable coating conditions are most frequently observed in the upper and lower end of the burning zone. This is clearly supported by the fact that most rotary kilns experience the most frequent refractory failures in these two critical areas. It should be noted that since the upper and lower burning zones are also within the vicinity of the first and second tires, brick failures are not only the result of variations in burning-zone conditions but, are also often the direct result of excessive tire clearance and shell ovality. Both the frequent falling out of coatings in these areas and the formation of too much coating can lead to troublesome ring formations.
Ring formations in the lower-transition zone (i.e., at the kiln discharge) are referred to as nose rings. Others refer to these as ash rings when the kiln is coal fired. Ring formations in the upper-transition zone are referred to as clinker rings. These ring formations can in many instances be so severe that they force operators to shut down the kiln and shoot these rings out with an industrial gun. The Cardox system has been successfully used for many years in Europe on several kilns to remove such rings. These devices are affixed to the kiln shell in strategic locations and use C02 cartridges to blast the rings while the kiln has only to be stopped for a short interval to load and trigger the cartridges.
Much research work has been done on the probable causes of these ring formations in the burning zone. The possible causes are many and no one single factor has yet been found that would be the main cause for all the rings formed. What seems to be true for one particular kiln might be compeltely wrong for another kiln. This is clearly explained in the fol lowing example: On many coal-fired kilns, operators have found a relationship between the fusion temperature of the coal ash and the frequency of ring formation. There appears to be more ring formation when the fusion temperature is low, i.e., when the ash contains larger amounts of fluxing iron and aluntina and less silica. However, this could not be the only cause for such ring formations because natural gas- and oil-fired kilns, which have no ash deposits in the burning zone, can have just as many ring problems as the coal-fired kilns. Hence, solutions for the elimination of rings in the burning zone are predontinantly found by a process of elimination. First, all probable causes are listed and then each suspected cause is eliminated or changed until hopefully an answer is found. From personal experience, the author has found the following factors to be possible contributors to ring formations in the burning zone:
POSSIBLE CAUSES FOR RING FORMATION IN THE BURNING ZONE
- Coal fineness too coarse.
- Low fusion temperature of coal ash.
- Kiln feed high on liquid content (silica, AIF ratios as well as lime saturation factor low).
- Incomplete calcination of the feed as it enters the burning zone (frequent dust flushes into and poor calcining conditions behind the burning zone).
- Frequent changes in chemical composition and fineness of kiln feed.
- Excessive dust generation in the cooler and burning zone (including changes in dust insufflation rates on wet-process kilns).
- Kiln speed too slow and feed loading too high in normal operation.
- Excessive variations of flame temperature and length during normal operation.
- Frequent changes in secondary air temperatures.
- Excessive frequency of kiln-operating upsets (burning zone temperature and location varies too frequently and by too large a range).
- Increased volatility of, and frequent changes in, alkali andsulfur contents in the fuel and feed.
Others have found other reasons for ring formation in the burning zone. Thus, the list could possibly be expanded to over 30.
It is of interest that half of the cited factors can be somehow controlled by the kiln operator and action taken to stabilize the flame and the kiln operation that might be beneficial in lessening the frequency of ring formation.
COATING AND RING FORMATION UPHILL OF THE BURNING ZONE
Less frequent but nevertheless equally troublesome are the so-called feed rings that form in the calcining zone of the rotary kiln. Wet-process kilns often experience so-called mudring formations in the chain section too. Finally, many preheater kilns and Lepol kilns experience ring formations and build-up problems at the feed inlet and in the lower preheat cyclone stage.
In investigations on this subject it has been found that the majority of these rings and heavy coatings in dry- and wet-process kilns are associated with one of the following factors:
- Internal cycle of the volatile constituents from the kiln feed and fuel (alkalies, sulfur, chlorides).
- Kiln-feed fineness.
- Irregular and insufficient control (frequent fluctuations) of the feed end temperature and kiln draft.
- Excessive dust generation within the rotary kiln proper.
Analysis of the materials from these rings or excessive coating buildu invariably showed high contents of calcium sulfates, potassium chloride and/or alkali sulfates. Efforts to alter the internal and external cycle t volatile components in the gas or feed stream have in many instance resulted in less frequent ring formations. Although the aforementione reasons apply to dry- and wet-process kilns, there have been many repor by others that have found a similar relationship in preheater and Lept kilns.
In one case of a wet-process kiln, it was found that mudring formatio was caused by a large percentage of the coarse fraction in tile kiln fee which contained predominantly free silica. In another case, mudrings wei initiated by the kiln operator who did not excercise sufficiently tight cor trol over the feed-end temperature, i.e, he allowed the temperature to var within a large range. Dust insufnation of the introduction of dust throur scoop feeders below tile chain section can also be a primary cause of, mudring formations in wet-process kilns. Last but not least, mudring) were also allowed to be formed simply by having the wrong type of chain system design or chain-link sizes.
References
- Waddell, Joseph 1962. Practical Quality Control for Concrete.New York:McGraw-Hill Book Co.
- Bogue, R.H. 1929. lru!ustrial Engineering Chemistry, Anal. Ed.1:192.
- Lea, F.M. and Parker, T.W. 1935. Building Research, Technical Paper 16, London.
- Kuehl, H.1929. Zemcnl, 18, 833.
- Lea, M. and Desch, C.H. 1935. The Chemistry ( Cemcnl ami Concrete·. Longmans, qrccn & Co.
click here to Download the Most Important 13 Books in Cement Industry
click here to Download the Most Important 13 Books in Cement Industry