Contents
The Importance of Sulphate Modulus in Kiln Operation: Managing Alkalis, Sulphur and Chlorides for Optimal Clinker Quality
If you want to download material books and sheets and documents will make you professional in cement engineering buy now
[wpecpp name=”Package ” price=”249.99″ align=”center”]
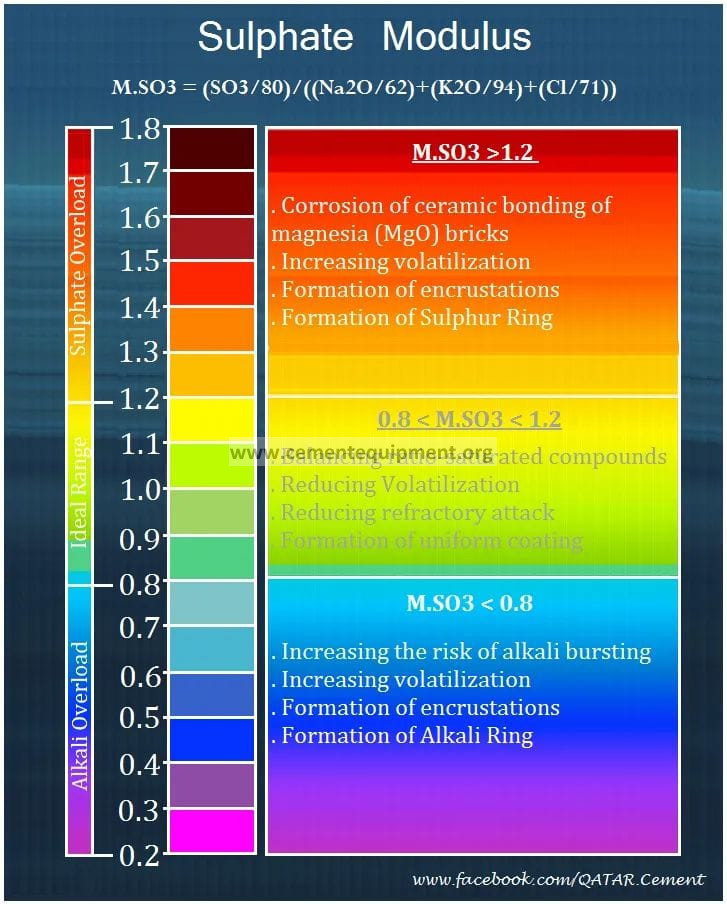
The Sulphate Modulus or Sulphur/ Alkali Ratio is a crucial parameter used in the operation of kilns. The S/A ratio is calculated in two ways: to measure the molar balance between the total inputs of sulphur and alkalis contributed by all raw materials, fuels, and AFR streams entering the kiln, and to measure the instantaneous molar sulphur/alkali balance in the kiln/preheater system based on hot meal. The equation used for both calculations is S/A = (SO3/80)/((Na2O/62) + (K2O/94) – (Cl/71)). However, when there is little or no chloride in the raw material and fuel inputs to the kiln, the chloride component is often ignored when calculating the S/A ratio of the total inputs.
The hot meal S/A ratio is calculated by subtracting chloride from the total alkali because alkali chlorides are more volatile than alkali sulphates and recirculate within the kiln. Over 98% of alkali chlorides, particularly KCl, are re-evaporated in the high temperature of the burning zone and return to the kiln inlet with the kiln gases. They condense on the incoming hot meal and continue to recycle. Any K2O or Na2O tied up with the chlorides are therefore not considered in the S/A ratio calculation for hot meal. The purpose of the hot meal S/A ratio is to predict the likelihood of alkali or sulphur-related buildups in the kiln inlet. Sudden increases in this ratio can indicate a lack of oxygen in the back end of the kiln and impending sulphur buildups, while a low value indicates an excess of alkalis. The portion of the alkalis that do not combine with SO3 to form sulphates will also recirculate in the kiln, increasing the potential for rings and preheater buildups.
When applied to the kiln material inputs, the sulphur/alkali ratio is used to manage raw material, fuel, and AFR inputs. New raw materials, fuels, and AFRs should be chosen, taking their effect on the overall sulphur/alkali ratio into consideration. Similarly, selective mining can “fine-tune” the chemistry of existing raw materials to optimize the raw mix sulphur/alkali ratio if it is not within the range 0.8 –> 1.2.
If alkalis are very high and are not balanced by sulphur, it will be very difficult for them to exit the kiln. They will continue to recirculate within the kiln/preheater system and increase the probability of kiln rings and preheater buildups. Clinker quality may suffer because free alkalis can enter into solid solution within the clinker minerals affecting their reactivity.
If sulphur is very high and is not balanced by alkalis, it will also continue to recirculate within the kiln/preheater system and increase the probability of kiln rings and preheater buildups. Excess sulphur in the hot meal can also form sulphospurrite (2.(CaO).SiO2.CaS04) in the middle cyclones, which forms exceedingly hard and dense buildups that can take a long time to remove. Clinker quality would also suffer because sulphur which is not combined with alkalis forms a solid solution with the silicate minerals, particularly C2S (up to 2%). Sulphur incorporated in this way stabilizes C2S and inhibits its reaction with CaO to form C3S. As a result, C2S content is increased, and C3S content is decreased in the clinker,
=======================
Sulphate Modulus Sulphate Modulus or Sulphur/ Alkali Ratio is used in two ways in kiln operation. The first is to measure if there is a molar balance between the total inputs of sulphur and alkalis contributed by all of the raw materials, fuels and AFR streams entering the kiln. The second is to measure the instantaneous molar sulphur/ alkali balance in the kiln/ preheater system (based on hot meal). Both use the same equation: S/A = (SO3/80)/ ((Na2O/62) + (K2O/94) – (Cl/71)) However, if there is little or no chloride in the raw material and fuel inputs to the kiln, the chloride component is often ignored when calculating the S/A ratio of the total inputs. For hot meal though, chloride is always subtracted from the total alkali because alkali chlorides are far more volatile than alkali sulphates and recirculate within the kiln. (Over 98% of alkali chlorides (particularly KCl) are re-evaporated in the high temperature of the burning zone and return to the kiln inlet with the kiln gases where they condense on the incoming hot meal and continue to recycle). Any K2O or Na2O tied up with the chlorides are therefore not considered in the S/A ratio calculation for hot meal. The purpose of the hot meal S/A ratio is to predict the likelihood of alkali or sulphur related buildups in the kiln inlet. In particular, sudden increases in this ratio can indicate lack of oxygen in the back end of the kiln and impending sulphur buildups. A low value indicates an excess of alkalis. The portion of the alkalis which do not combine with SO3 to form sulphates will also recirculate in the kiln, increasing the potential for rings and preheater buildups. When applied to the kiln material inputs, the sulphur/ alkali ratio is used to manage raw material, fuel and AFR inputs. New raw materials, fuels and AFRs should be chosen taking their effect on the overall sulphur/ alkali ratio into consideration. Similarly, selective mining can “fine tune” the chemistry of existing raw materials to optimise the raw mix sulphur/ alkali ratio, if it is not within the range 0.8 –> 1.2 If alkalis are very high and are not balanced by sulphur, it will be very difficult for them to exit the kiln. They will therefore continue to recirculate within the kiln/preheater system and increase the probabliity of kiln rings and preheater buildups. Clinker quality may suffer because free alkalis can enter into solid solution within the clinker minerals affecting their reactivity. If sulphur is very high and is not balanced by alkalis, it will also continue to recirculate within the kiln/preheater system and increase the probabliity of kiln rings and preheater buildups. Excess sulphur in the hot meal can also form sulphospurrite (2.(CaO).SiO2.CaS04) in the middle cyclones, which forms exceedingly hard and dense buildups which can take a long time to remove. Clinker quality would also suffer because sulphur which is not combined with alkalis forms a solid solution with the silicate minerals, particularly C2S (up to 2%). Sulphur incorporated in this way stabilises C2S and inhibits its reaction with CaO to form C3S. As a result, C2S content is increased and C3S content is decreased in the clinker, causing a reduction in cement strengths. If chloride is very high, it will first combine with all of the alkalis present forming alkali chlorides which will recirculate in the kiln and increase the probability of buildups in the preheater. Any remaining chloride will then combine with CaO to form CaCl2 which has a very low melting point (770-780 C). This will make the hot meal extremely “sticky” at this temperature and increase the chance of buildups higher up the preheater. Chlorides also form eutectic mixtures with sulphates of potassium, sodium, calcium and magnesium. These eutectic mixtures have melting points much lower than that of the pure compounds, further increasing the likelyhood of rings and buildups. Above 0.015%, in the raw meal, chloride recirculation is so bad that blockages in the preheater are eventually inevitable. If the chloride does manage to escape the kiln(ie during kiln trips, stoppages etc), too much chloride in the clinker can accelerate the corrosion of reinforcing steel in the concrete